OraSure
Technologies Receives FDA Approval for Its OraQuick(R) HCV Rapid
Test Using Fingerstick Whole Blood
Bethlehem,
Pa. -- February 22, 2011 -- OraSure Technologies, Inc. (Nasdaq:OSUR)
announced today that its OraQuick HCV Rapid Antibody Test has
now been approved by the U.S. Food and Drug Administration ("FDA")
for use in detecting HCV antibodies with a fingerstick whole
blood sample. The fingerstick whole blood claim is the second
application to be approved by the FDA for the OraQuick(R) HCV
test. The product received an initial approval for use in persons
at risk for HCV infection with venous whole blood specimens
in June 2010.
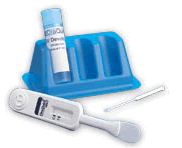 The
OraQuick HCV Rapid Antibody Test is the only rapid, point-of-care
test for the detection of antibodies to the hepatitis C virus
that is approved by the FDA. The test, which utilizes the OraQuick
technology platform, provides results in 20 minutes. The
OraQuick HCV Rapid Antibody Test is the only rapid, point-of-care
test for the detection of antibodies to the hepatitis C virus
that is approved by the FDA. The test, which utilizes the OraQuick
technology platform, provides results in 20 minutes.
"Receiving FDA approval to test individuals at risk using
a fingerstick whole blood sample significantly expands the flexibility
and versatility of our OraQuick Rapid HCV Antibody Test,"
said Douglas A. Michels, President and Chief Executive Officer
of OraSure Technologies. "By eliminating the need for a
blood draw, healthcare providers will be able to identify more
individuals infected with hepatitis C and get them into care."
There are an estimated 4.1 million Americans, or 1.6 percent
of the U.S. population, that are or have been infected with
HCV. New infections in the U.S. are estimated at approximately
20,000 per year. On a worldwide basis, there are an estimated
180 million people who are chronically infected with HCV, with
an estimated 3 to 4 million individuals newly infected each
year.
The
company previously received approval to affix the CE mark to
its OraQuick HCV test in December 2009 for use with oral fluid,
fingerstick whole blood, venous whole blood, serum and plasma.
The CE Mark was required in order to sell the product in the
European Union.
As previously announced, OraSure has entered into agreements
with Merck & Co. (NYSE:MRK), through its predecessor Schering
Plough Corp., to collaborate on the development and promotion
of the OraQuick HCV test. Under these agreements, Merck will
provide detailing and other promotional support for the test
in the physicians' office markets in the United States and internationally.
About OraSure Technologies
 OraSure
Technologies develops, manufactures and markets oral fluid specimen
collection devices using proprietary oral fluid technologies,
diagnostic products including immunoassays and other in vitro
diagnostic tests, and other medical devices. These products
are sold in the United States as well as internationally to
various clinical laboratories, hospitals, clinics, community-based
organizations and other public health organizations, distributors,
government agencies, physicians' offices, and commercial and
industrial entities. OraSure Technologies is the leading supplier
of oral-fluid testing solutions for drugs of abuse and for the
detection of antibodies to HIV. For more information on the
company, please go to www.orasure.com. OraSure
Technologies develops, manufactures and markets oral fluid specimen
collection devices using proprietary oral fluid technologies,
diagnostic products including immunoassays and other in vitro
diagnostic tests, and other medical devices. These products
are sold in the United States as well as internationally to
various clinical laboratories, hospitals, clinics, community-based
organizations and other public health organizations, distributors,
government agencies, physicians' offices, and commercial and
industrial entities. OraSure Technologies is the leading supplier
of oral-fluid testing solutions for drugs of abuse and for the
detection of antibodies to HIV. For more information on the
company, please go to www.orasure.com.
2/25/11
Source
OraSure
Technologies. OraSure Technologies Receives FDA Approval for
Its OraQuick(R) HCV Rapid Test Using Fingerstick Whole Blood.
Press release. February 22, 2011.
|
| |
|
|
